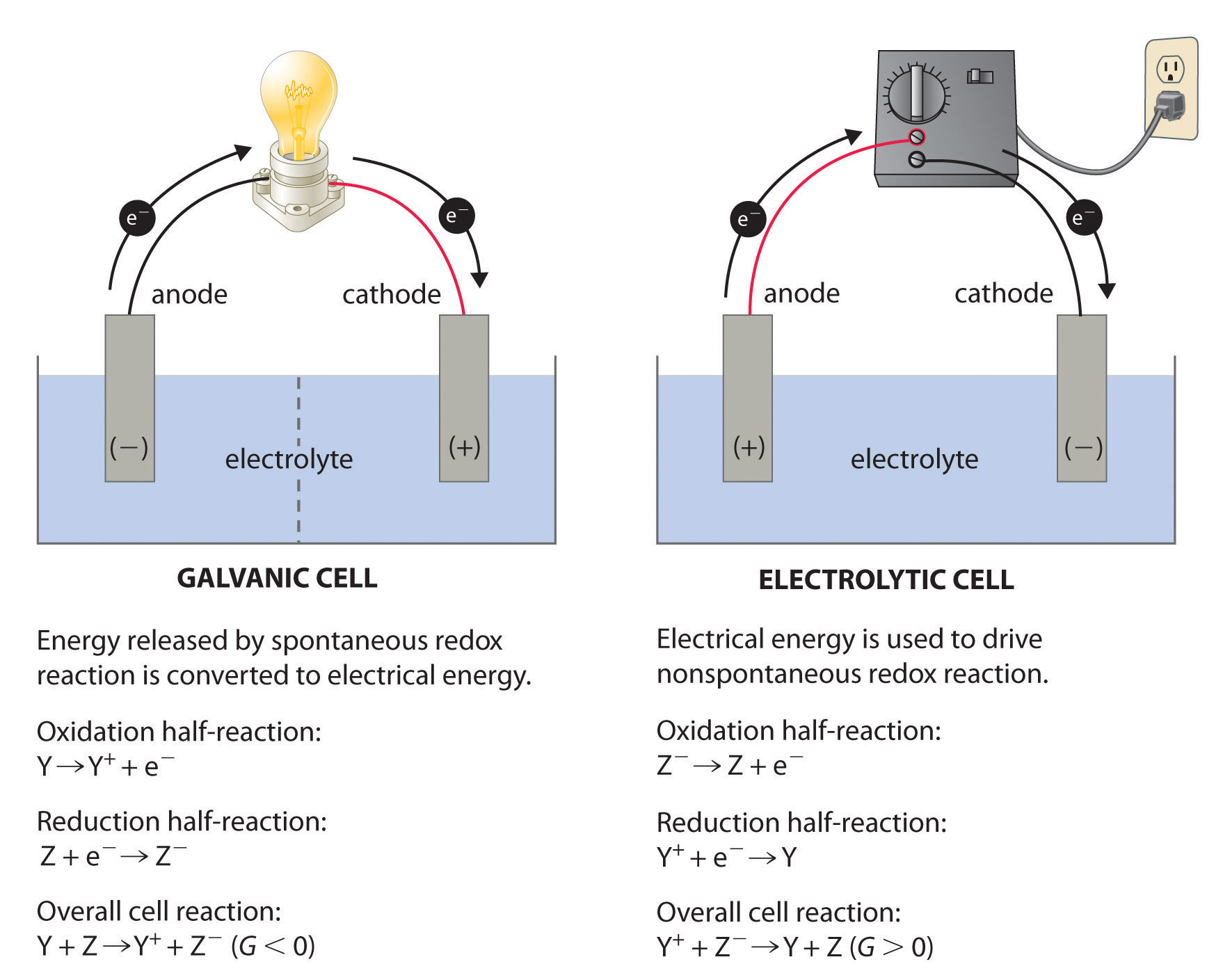
What Is Electrochemical Corrosion Used For . In essence, electrochemistry and corrosion science are the main topics used in this book for characterizing metal oxidation known. Electrochemical corrosion of metals occurs when electrons from atoms at the surface of the metal are transferred to a suitable electron acceptor. Electrochemical corrosion, according to text books, needs four elements: Electrochemical corrosion refers to the process of corrosion that occurs due to the presence of an anode (corroding metal), a cathode (metal or. Three essentials of electrochemical corrosion. We also examine the chemical basis for some common methods for. In the process of electrochemical corrosion, metal atoms lose electrons to become ions and in the process they go into solution. In this section, we describe some of the chemical and electrochemical processes responsible for corrosion.
from rohanfersmorrison.blogspot.com
We also examine the chemical basis for some common methods for. In this section, we describe some of the chemical and electrochemical processes responsible for corrosion. Electrochemical corrosion of metals occurs when electrons from atoms at the surface of the metal are transferred to a suitable electron acceptor. Three essentials of electrochemical corrosion. In the process of electrochemical corrosion, metal atoms lose electrons to become ions and in the process they go into solution. Electrochemical corrosion, according to text books, needs four elements: Electrochemical corrosion refers to the process of corrosion that occurs due to the presence of an anode (corroding metal), a cathode (metal or. In essence, electrochemistry and corrosion science are the main topics used in this book for characterizing metal oxidation known.
Identify the Conditions for a Standard Electrochemical Cell.
What Is Electrochemical Corrosion Used For We also examine the chemical basis for some common methods for. We also examine the chemical basis for some common methods for. In essence, electrochemistry and corrosion science are the main topics used in this book for characterizing metal oxidation known. Electrochemical corrosion refers to the process of corrosion that occurs due to the presence of an anode (corroding metal), a cathode (metal or. In the process of electrochemical corrosion, metal atoms lose electrons to become ions and in the process they go into solution. Electrochemical corrosion, according to text books, needs four elements: In this section, we describe some of the chemical and electrochemical processes responsible for corrosion. Three essentials of electrochemical corrosion. Electrochemical corrosion of metals occurs when electrons from atoms at the surface of the metal are transferred to a suitable electron acceptor.
From www.youtube.com
Corrosion Electrochemical Cell or Corrosion Cell (Chapter 3 What Is Electrochemical Corrosion Used For In essence, electrochemistry and corrosion science are the main topics used in this book for characterizing metal oxidation known. Electrochemical corrosion of metals occurs when electrons from atoms at the surface of the metal are transferred to a suitable electron acceptor. Three essentials of electrochemical corrosion. Electrochemical corrosion, according to text books, needs four elements: In the process of electrochemical. What Is Electrochemical Corrosion Used For.
From www.youtube.com
12. Electrochemical theory of corrosion and prevention What Is Electrochemical Corrosion Used For Electrochemical corrosion, according to text books, needs four elements: Three essentials of electrochemical corrosion. Electrochemical corrosion refers to the process of corrosion that occurs due to the presence of an anode (corroding metal), a cathode (metal or. In essence, electrochemistry and corrosion science are the main topics used in this book for characterizing metal oxidation known. We also examine the. What Is Electrochemical Corrosion Used For.
From www.researchgate.net
Electrochemical accelerated corrosion method (a) Schematic diagram of What Is Electrochemical Corrosion Used For In the process of electrochemical corrosion, metal atoms lose electrons to become ions and in the process they go into solution. In essence, electrochemistry and corrosion science are the main topics used in this book for characterizing metal oxidation known. We also examine the chemical basis for some common methods for. Electrochemical corrosion refers to the process of corrosion that. What Is Electrochemical Corrosion Used For.
From www.researchgate.net
Scheme illustrating (a) the electrochemical corrosion process at a What Is Electrochemical Corrosion Used For Electrochemical corrosion of metals occurs when electrons from atoms at the surface of the metal are transferred to a suitable electron acceptor. In the process of electrochemical corrosion, metal atoms lose electrons to become ions and in the process they go into solution. Electrochemical corrosion refers to the process of corrosion that occurs due to the presence of an anode. What Is Electrochemical Corrosion Used For.
From knowledge.electrochem.org
Electrochemistry Encyclopedia Electrochemistry of corrosion What Is Electrochemical Corrosion Used For In the process of electrochemical corrosion, metal atoms lose electrons to become ions and in the process they go into solution. In essence, electrochemistry and corrosion science are the main topics used in this book for characterizing metal oxidation known. Electrochemical corrosion of metals occurs when electrons from atoms at the surface of the metal are transferred to a suitable. What Is Electrochemical Corrosion Used For.
From www.myxxgirl.com
Electrochemistry Class Chemistry Commercial Cells And Corrosion My What Is Electrochemical Corrosion Used For In this section, we describe some of the chemical and electrochemical processes responsible for corrosion. In the process of electrochemical corrosion, metal atoms lose electrons to become ions and in the process they go into solution. Electrochemical corrosion of metals occurs when electrons from atoms at the surface of the metal are transferred to a suitable electron acceptor. Electrochemical corrosion. What Is Electrochemical Corrosion Used For.
From minemaster381.weebly.com
Fundamentals Electrochemical Corrosion minemaster What Is Electrochemical Corrosion Used For In the process of electrochemical corrosion, metal atoms lose electrons to become ions and in the process they go into solution. In essence, electrochemistry and corrosion science are the main topics used in this book for characterizing metal oxidation known. Three essentials of electrochemical corrosion. Electrochemical corrosion, according to text books, needs four elements: We also examine the chemical basis. What Is Electrochemical Corrosion Used For.
From present5.com
LECTURE 10 REDOX AND ELECTROCHEMISTRY Figure 4 What Is Electrochemical Corrosion Used For In this section, we describe some of the chemical and electrochemical processes responsible for corrosion. In essence, electrochemistry and corrosion science are the main topics used in this book for characterizing metal oxidation known. Electrochemical corrosion of metals occurs when electrons from atoms at the surface of the metal are transferred to a suitable electron acceptor. Electrochemical corrosion refers to. What Is Electrochemical Corrosion Used For.
From www.youtube.com
Hydrogen evolution corrosion YouTube What Is Electrochemical Corrosion Used For Three essentials of electrochemical corrosion. Electrochemical corrosion, according to text books, needs four elements: We also examine the chemical basis for some common methods for. Electrochemical corrosion of metals occurs when electrons from atoms at the surface of the metal are transferred to a suitable electron acceptor. In the process of electrochemical corrosion, metal atoms lose electrons to become ions. What Is Electrochemical Corrosion Used For.
From www.studypool.com
SOLUTION Electrochemistry and corrosion Studypool What Is Electrochemical Corrosion Used For Electrochemical corrosion of metals occurs when electrons from atoms at the surface of the metal are transferred to a suitable electron acceptor. In the process of electrochemical corrosion, metal atoms lose electrons to become ions and in the process they go into solution. Three essentials of electrochemical corrosion. Electrochemical corrosion, according to text books, needs four elements: In essence, electrochemistry. What Is Electrochemical Corrosion Used For.
From www.slideserve.com
PPT Corrosion PowerPoint Presentation, free download ID2790471 What Is Electrochemical Corrosion Used For Electrochemical corrosion refers to the process of corrosion that occurs due to the presence of an anode (corroding metal), a cathode (metal or. In the process of electrochemical corrosion, metal atoms lose electrons to become ions and in the process they go into solution. Electrochemical corrosion of metals occurs when electrons from atoms at the surface of the metal are. What Is Electrochemical Corrosion Used For.
From www.studypool.com
SOLUTION Type of electrochemical corrosion Studypool What Is Electrochemical Corrosion Used For In this section, we describe some of the chemical and electrochemical processes responsible for corrosion. In essence, electrochemistry and corrosion science are the main topics used in this book for characterizing metal oxidation known. Electrochemical corrosion of metals occurs when electrons from atoms at the surface of the metal are transferred to a suitable electron acceptor. Electrochemical corrosion, according to. What Is Electrochemical Corrosion Used For.
From www.comsol.it
Modeling Pitting Corrosion in COMSOL Multiphysics® COMSOL Blog What Is Electrochemical Corrosion Used For Three essentials of electrochemical corrosion. In this section, we describe some of the chemical and electrochemical processes responsible for corrosion. We also examine the chemical basis for some common methods for. In the process of electrochemical corrosion, metal atoms lose electrons to become ions and in the process they go into solution. Electrochemical corrosion refers to the process of corrosion. What Is Electrochemical Corrosion Used For.
From www.youtube.com
Corrosion in Electrochemistry Electrochemistry Chemistry Class 12 What Is Electrochemical Corrosion Used For In essence, electrochemistry and corrosion science are the main topics used in this book for characterizing metal oxidation known. Three essentials of electrochemical corrosion. In the process of electrochemical corrosion, metal atoms lose electrons to become ions and in the process they go into solution. Electrochemical corrosion of metals occurs when electrons from atoms at the surface of the metal. What Is Electrochemical Corrosion Used For.
From www.researchgate.net
Representation of Electrochemical Corrosion Process. Download What Is Electrochemical Corrosion Used For In this section, we describe some of the chemical and electrochemical processes responsible for corrosion. Electrochemical corrosion refers to the process of corrosion that occurs due to the presence of an anode (corroding metal), a cathode (metal or. In essence, electrochemistry and corrosion science are the main topics used in this book for characterizing metal oxidation known. Electrochemical corrosion of. What Is Electrochemical Corrosion Used For.
From www.hilti.com
Corrosion in Construction Hilti USA What Is Electrochemical Corrosion Used For Three essentials of electrochemical corrosion. Electrochemical corrosion of metals occurs when electrons from atoms at the surface of the metal are transferred to a suitable electron acceptor. We also examine the chemical basis for some common methods for. In the process of electrochemical corrosion, metal atoms lose electrons to become ions and in the process they go into solution. In. What Is Electrochemical Corrosion Used For.
From www.youtube.com
Hydrogen evolution and Oxygen Absorption Mechanism of wet corrosion What Is Electrochemical Corrosion Used For Three essentials of electrochemical corrosion. In this section, we describe some of the chemical and electrochemical processes responsible for corrosion. In essence, electrochemistry and corrosion science are the main topics used in this book for characterizing metal oxidation known. Electrochemical corrosion, according to text books, needs four elements: Electrochemical corrosion of metals occurs when electrons from atoms at the surface. What Is Electrochemical Corrosion Used For.
From mavink.com
Components Of Electrochemical Cell What Is Electrochemical Corrosion Used For Electrochemical corrosion, according to text books, needs four elements: We also examine the chemical basis for some common methods for. Electrochemical corrosion refers to the process of corrosion that occurs due to the presence of an anode (corroding metal), a cathode (metal or. Three essentials of electrochemical corrosion. In the process of electrochemical corrosion, metal atoms lose electrons to become. What Is Electrochemical Corrosion Used For.